A federal judge in Texas on Friday issued a ruling to revoke the Food and Drug Administration’s nearly 23-year-old approval of the safe and effective medication for abortion and miscarriage, mifepristone. While expected, the ruling questions the FDA’s authority over all drugs and threatens to weaken the country’s key development pipeline, industry leaders and legal experts say.
In a public letter circulated this weekend, biotechnology and pharmaceutical industry executives and leaders condemned the ruling and called for it to be reversed along with “due restitution” from the FDA’s authority.
As of Monday afternoon, the letter had about 400 signatures and counting. Among them are major players in the industry, including Pfizer CEO Albert Bourla; Alisha Alaimo, President of Biogen; Christopher Tan, an executive for Merck & Co.; Imran Nasrullah, vice president of Bayer Pharmaceuticals; and a senior clinical leader at Novartis, Nancy Lewis. But the vast majority will come from smaller biotech companies, which will lose most of the downstream effects of the ruling issued by District Judge Matthew Kacsmaryk.
In the letter, biopharmaceutical leaders rebuked Kacsmaryk as an activist judge, emphasizing that he has “no scientific training” and that his ruling “ignores decades of scientific evidence,” finding that mifepristone is “safer than Tylenol, nearly all antibiotics and insulin”. The ruling also fundamentally undermined the FDA’s authority to approve and regulate safe, effective drugs for Americans, creating “uncertainty for the entire biopharmaceutical industry.”
Less drugs, less innovation
That uncertainty, they say, will deter investment in drug development and jeopardize innovation. The industry is already “inherently risky,” they note, referring to the fact that most drugs fail to successfully pass clinical trials despite requiring millions of dollars in funding over years, and often decades, of development. With the few drugs that do make it to market, drugmakers rely on years of profit from sales that can help support riskier research and development programs.
The biopharmaceutical industry in the US is unique in size, productivity and innovation. It produces the most FDA-approved drugs of any country, and has some of the highest percentages of drugs with novel structures and mechanisms. The US is also an outlier in producing more drugs for unmet medical needs than others and relies more on biotech companies and academic institutions than just pharmaceutical companies.
But that superiority depends on a reliable regulatory process, which is now in question with last week’s mifepristone ruling. “Legal activism will not stop here,” the biopharmaceutical leaders wrote. “If courts can overturn drug approvals without regard to science or evidence, or the complexity required to fully investigate the safety and efficacy of new drugs, then any drug faces the same risk as mifepristone,” they said.
The leaders acknowledge that the FDA’s processes and oversight are “not perfect” but “have resulted in decades of unsurpassed medical innovation.”
In a statement Friday, President Joe Biden reiterated their concerns about the broader implications of the ruling. “If this ruling were to hold, there will be virtually no FDA-approved prescription that is safe from this kind of political, ideological attack,” the president said.
Legal experts have said the same. “If your approval can be revoked in the blink of an eye by a single judge, that’s really kind of scary,” I. Glenn Cohen, a Harvard Law School professor and bioethics expert, told The New York Times.
Safe, effective, but not the only option
The Justice Department, which represents the FDA, is appealing the ruling. The FDA released a statement Friday saying, “The FDA stands by its determination that mifepristone is safe and effective under its approved terms of use for medical termination of early pregnancy, and believes that patients should have access to FDA-approved medications that the FDA has determined to be safe and effective for its intended use.”
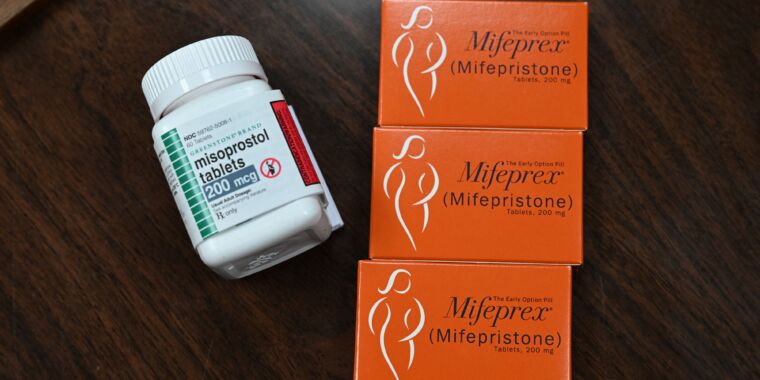
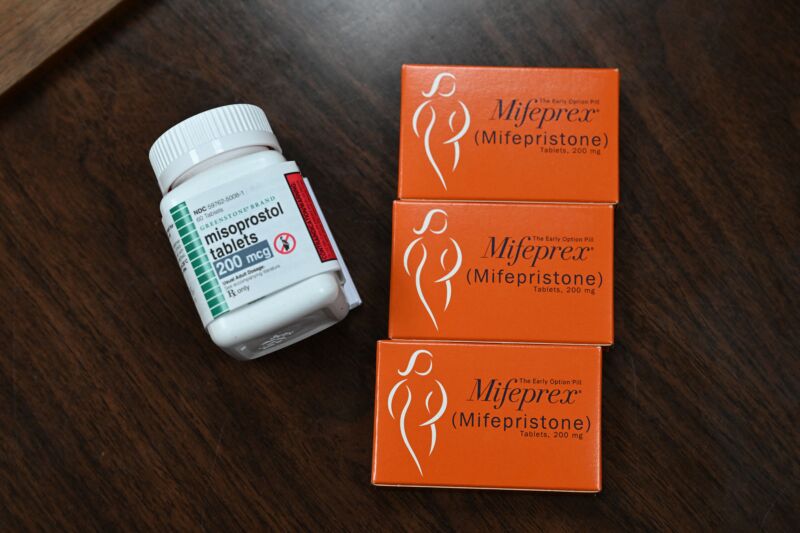
For now, mifepristone remains available. It is used in combination with another medicine called misoprostol to terminate an early pregnancy. The FDA has approved its use in the first 10 weeks (70 days) of pregnancy. (Pregnancy is dated from the first day of the last menstrual period, not an approximate day of conception, which is about two weeks after the first day of the last menstrual period, or when.) However, the World Health Organization says the regimen is safe up to 12 weeks (84 days) of a pregnancy.
Mifepristone is a synthetic steroid that blocks progesterone, which plays a vital role in maintaining a pregnancy by controlling the environment of the uterus. Misoprostol is a synthetic prostaglandin (a hormone-like substance) that causes contractions and dilation of the cervix. The result of the regimen is cramping, bleeding and termination of pregnancy. Medical abortion is used in more than half of abortions in the US.
If mifepristone is no longer available in the US, clinicians are willing to use a misoprostol-only protocol, which is commonly used outside the US. It is safe, effective and works faster, but it usually has more side effects, such as nausea, diarrhea and vomiting. But with Roe against Wade Although reproductive health care was rolled back in June last year, it remains under constant and serious threat in the US. Currently, 12 states enforce a total ban on abortion, and many others have a range of restrictions.