With the country dealing with a relatively large summer surge of COVID-19, the Food and Drug Administration is considering approving this year's COVID-19 vaccines targeted at the virus as early as this week, according to a CNN report citing anonymous officials familiar with the matter.
Last year, the FDA greenlit the 2023-2024 COVID vaccines on September 11, close to the peak of SARS-CoV-2 transmission in that year’s summer surge. This year’s summer surge has started earlier and, by some metrics, is peaking at much higher levels than in previous years.
Currently, detection of SARS-CoV-2 in wastewater is showing “very high” levels of the virus in 32 states and the District of Columbia. Another 11 states are listed as having “high” levels. Looking at trends, the southern and western regions of the country are currently reporting SARS-CoV-2 levels in wastewater that rival the winter waves of 2022-2023 and 2023-2024, both of which peaked in late December.
-
SARS-CoV-2 levels in wastewater, by state
-
Trends in SARS-CoV-2 levels in wastewater by region over the past year
-
Trends in SARS-CoV-2 levels in wastewater by region for the entire pandemic
Test positivity, a metric that has weakened due to the dramatic drop in testing, shows a weekly test positivity rate of 18.1 percent for mid-August (on a testing volume of about 43,000). Such a rate, if it is truly reflective of cases, has not been seen since the first skyrocketing omicron wave of January 2022, which peaked at 30.5 percent (on a testing volume of about 991,000).
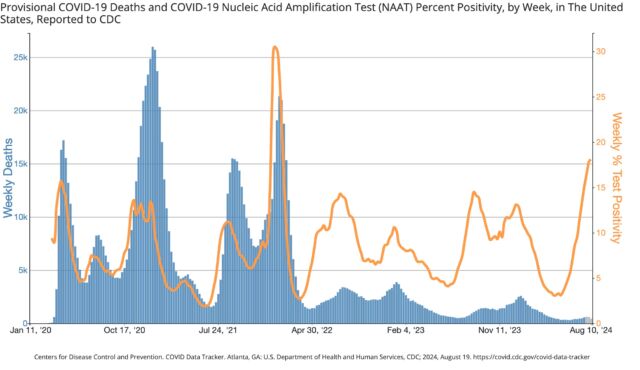
The good news is that, given the significant accumulation of protection from prior infections and vaccinations, the two most serious metrics—emergency department visits and deaths—have not seen similar increases. The weekly percentage of emergency department visits with a COVID-19 diagnosis is low and comparable to last year’s summer surge. Deaths are also low, though still only preliminary numbers for the most recent weeks.
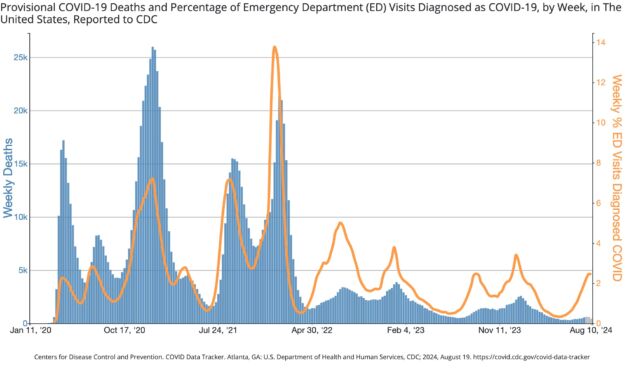
The FDA has embraced a strategy of offering COVID-19 vaccines annually in anticipation of winter, not summer, waves. The agency’s thinking has always been to encourage Americans to get their flu and COVID-19 vaccines together between September and November, just before a flurry of cold-respiratory illnesses gathers. The new boost in vaccinations could reduce levels of severe respiratory illness at a time when health care systems are most at risk of being overwhelmed.
Seasonality
But while seasonal flu and some other respiratory infections occur almost exclusively in the winter, COVID-19’s seasonality has never been a given. And so far, summer waves have been just as consistent as winter waves, causing some inconvenience as vaccines roll out.
Some experts have recommended getting a COVID-19 vaccine to protect against the summer surge. “Now is the time to get a dose with this surge,” Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, told CNN on Sunday.
However, the only vaccines currently available target last year’s strains (related to the XBB.1.5 omicron variant), which are long gone and may not provide strong protection against current strains (the JN.1 and KP.2 omicron variants). Even if the 2024-2025 KP.2-targeting vaccine is approved by the FDA this week and hits pharmacy shelves next week, it will take two weeks for one dose to provide full protection. By then, the summer surge will likely be waning. In fact, it appears to have already peaked in some parts of the country, including some southern and western areas.
The other thing to consider is the timing for maximum protection against the likely winter surge. For healthy people ages 5 and older, the CDC recommended last year that they get just one shot. The shots provide peak protection for about four months. If you get your annual shot in early September, your protection could wane if COVID-19 spikes again around New Year’s, as it has the past two years.
Under the 2023-2024 guidelines, people 65 and older can get a second COVID-19 booster four months after their first. People with moderately or severely weakened immune systems can also get additional doses of the updated COVID-19 vaccine.