An mRNA-based flu vaccine designed to provide long-term protection against a wide variety of flu viruses is now in a phase I clinical trial, the National Institutes of Health announced this week.
The trial brings the remarkable success of the mRNA vaccine platform to the long-standing effort to develop a universal flu vaccine. Currently, health systems around the world are fighting the seasonal blight with injections that must be reformulated each year to match the circulating species. This reformulation occurs months before the typical handover, giving manufacturers time to produce doses at scale, but also giving the voltage circulation opportunities to shift unexpectedly. If the Injection of the Year is a poor match for the strains circulating in a given season, its efficacy against infection may be abysmal. But even if the shot is well matched, people will need another shot next year.
“A universal flu vaccine would be a major public health achievement and could eliminate the need for both the annual development of seasonal flu vaccines and the need for patients to get a flu shot every year,” said Hugh Auchincloss, acting director of the NIHs. National Institute of Allergy and Infectious Diseases, said in a press release. “In addition, some strains of the flu virus have significant pandemic potential. A universal flu vaccine could serve as an important line of defense against the spread of a future flu pandemic.”
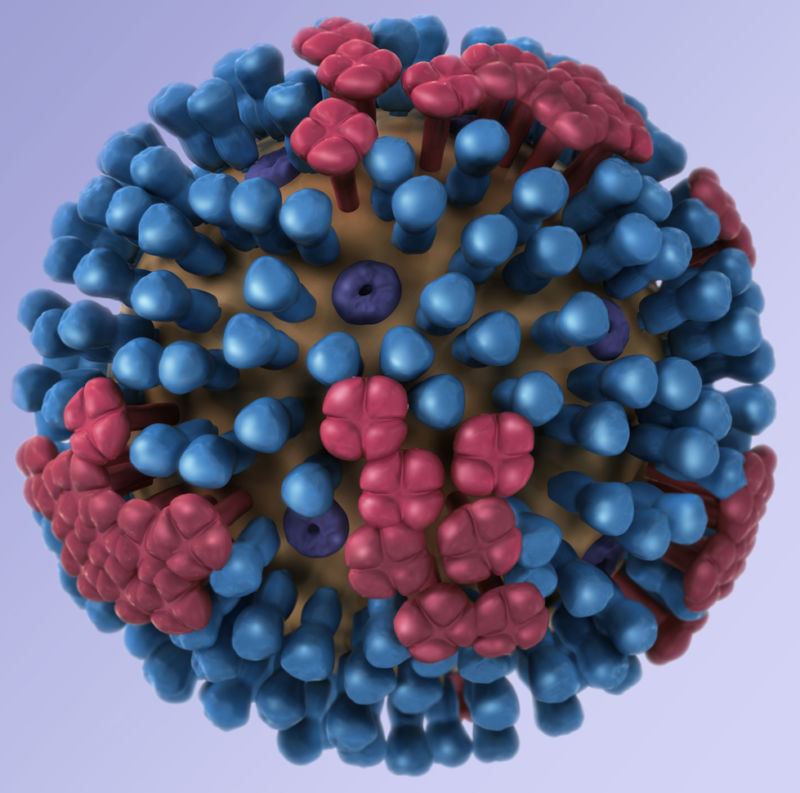
A successful design has proved elusive. Flu vaccines often generate immune responses to rapidly evolving bits of protein on the outside of flu virus particles, hemagglutinin (Ha or H) and neuraminidase (Na or N). These proteins are responsible for helping the virus break into and out of human cells, respectively, during an infection. Both proteins look a bit like lollipops stuck to the outside of the virus particle, with their buds evolving and important targets for powerful antibodies against the virus.
For the universal vaccine design, NIH researchers did not focus on the peaks of these proteins, but on part of the Ha protein’s stem – a highly conserved part of the protein that does not evolve as quickly. Human antibodies targeting this conserved region are likely to target Ha proteins from a range of different flu strains in the same class. And because this section doesn’t evolve that fast, the vaccine could produce long-lasting immunity. With this design, the mRNA-based vaccine will contain a piece of genetic code in the form of mRNA that gives human cells the blueprints for this conserved stem region. From there, the immune system can learn to target it.
There is already data suggesting that this target could work. Before using an mRNA-based design, NIH researchers developed a similar HA strain-targeted vaccine that appeared safe and effective in a phase I trial. The vaccine uses stabilized protein fragments from the Ha strain stuck onto a nanoparticle. Last month, NIH researchers published results showing that this nanoparticle vaccine induced cross-reacting neutralizing antibodies against influenza viruses in the same virus group (H1). And those neutralizing antibodies lingered for more than a year after vaccination. The vaccine candidate has moved on to a second trial. The researchers hope that having multiple platforms in the works will increase their chances of getting a successful vaccine.
For now, the mRNA-based vaccine will begin a small trial with just 50 people recruited through Duke University partners. Three groups of 10 volunteers are given different vaccine doses to find the optimal dose. Once that is found, 10 more will be vaccinated and their responses will be compared to a control group of 10 people who will receive a standard annual flu vaccine.

