Waipapa Taumata Rau/Auckland University
There’s rarely time to write about every cool science story that comes our way. That’s why we’re posting another special Twelve Days of Christmas series of posts this year, highlighting a science story that fell through the cracks in 2022 every day from December 25 through January 5. Today: Scientists in New Zealand and Australia created tiny metal snowflakes.
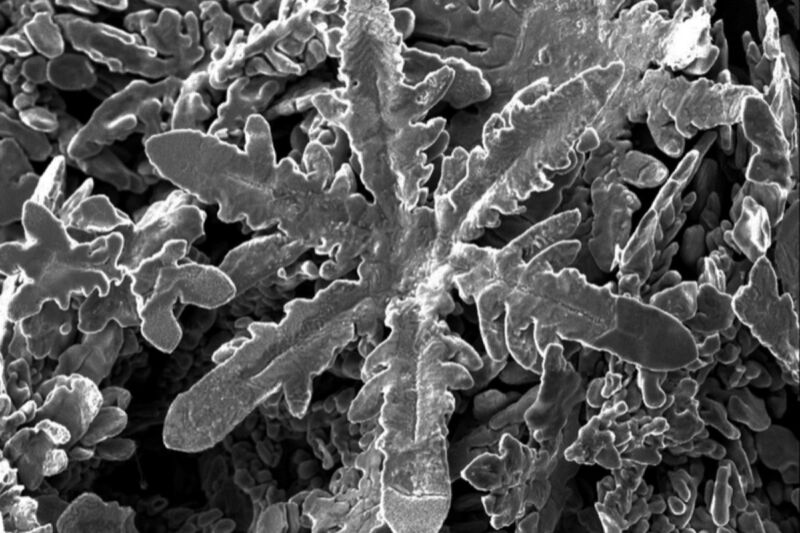
Scientists in New Zealand and Australia were conducting atomic-scale experiments with various metals dissolved in a liquid solvent of gallium when they noticed something unusual: different types of metal self-assembled into different shapes of crystals – with zinc creating tiny metallic snowflakes. They described their results in a paper published earlier this month in the journal Science.
“Unlike top-down approaches to forming nanostructures – by cutting away material – this bottom-up approach is based on atom self-assembly,” says study co-author Nicola Gaston of the University of Auckland. “This is how nature makes nanoparticles, and it’s both less wasteful and much more accurate than top-down methods. There’s also something really cool about making a metallic snowflake!”
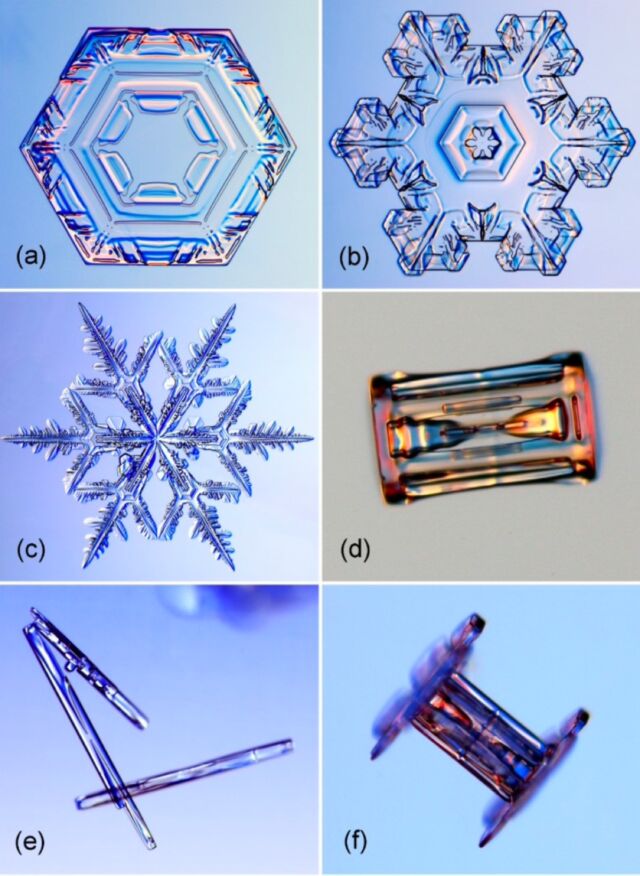
Snowflakes are the best known example of crystal growth, at least among the general population. It has long been known that water vapor can condense directly into small ice crystals under certain conditions, usually in the form of a hexagonal prism (two hexagonal “basal” faces and six rectangular “prism” faces). But that crystal also attracts more cooled water droplets in the air. Branches sprout from the corners of the single crystals, forming snowflakes of increasingly complex shapes.
The shapes of snowflakes and snow crystals have long fascinated scientists, such as Johannes Kepler, who in 1611 took a break from stargazing to publish a short paper entitled “On the Six-Cornered Snowflake.” He was intrigued by the fact that snow crystals always seem to have sixfold symmetry. Some 20 years later, Rene Descartes waxed poetic after observing much rarer 12-sided snowflakes, “so perfectly formed in hexagons, and whose six sides were so straight, and the six angles so equal, that it is impossible for men to to make something.” so precise.” He pondered how such a perfectly symmetrical shape could have come about, and finally arrived at a fairly accurate description of the water cycle, adding that “they were obliged to arrange themselves so that they were all surrounded by six others in the same plane. , according to the ordinary order of nature.”
Robert Hookes micrography, published in 1665, contained some sketches of snowflakes that he observed under his microscope. But no one conducted a truly systematic study of snow crystals until the 1950s, when a Japanese nuclear physicist named Ukichiro Nakaya identified and cataloged all major types of snow crystals. Nakaya was the first person to grow artificial snow crystals in the lab. In 1954 he published a book about his findings: Snow crystals: natural and artificial.
Watch as a snowflake “grows” into an intricate crystal structure. Credits: Kenneth Libbrecht
Thanks to Nakaya’s pioneering work, we know that certain atmospheric conditions, such as temperature and humidity, can affect the shape of a snowflake. Star-like shapes form at -2 degrees Celsius and -15 degrees Celsius, while columns form at -5 degrees Celsius and again at about -30 degrees Celsius. And the higher the humidity, the more complex the shape. If the humidity is particularly high, they can even form into long needles or large thin plates.
Caltech physicist Kenneth Libbrecht has been studying and photographing the formation of snowflakes for more than two decades. And like Nakaya, he also makes his own snowflakes in the lab, carefully using a small paintbrush to transfer the delicate textures onto a glass slide, taking pictures with a digital camera mounted on a high-resolution microscope. He has documented the many types of snow crystals over the years, culminating in a 540-page monograph that tour de force of snowflake physics.
Most recently, in 2019, Libbrecht developed what he called a “semi-empirical” model of the atomic processes at work to explain why there are two primary types of snowflakes: the iconic flat star, with six or twelve points, and a column, sometimes sandwiched by flat caps and sometimes resembling a bolt from a hardware store. Libbrecht wanted to investigate what exactly changes with the shifts in temperature. His model incorporates a phenomenon called surface energy-driven molecular diffusion. per quantity:
A thin, flat crystal (plate-like or star-like) forms when the edges bind into material faster than the two faces of the crystal. The nascent crystal will spread outward. However, when the faces grow faster than the edges, the crystal enlarges and forms a needle, hollow column, or bar. According to Libbrecht’s model, water vapor first settles on the corners of the crystal and then diffuses across the surface to the edge of the crystal or to the faces, causing the crystal to grow outward or upward, respectively. Which of these processes wins when different surface effects and instabilities interact depends largely on temperature.
Kenneth Libbrecht
With this latest work, Gaston and her colleagues extended the ice snowflake analogy to metals. They dissolved samples of nickel, copper, zinc, tin, platinum, bismuth, silver and aluminum in gallium, which liquefies just above room temperature, making it an excellent liquid solvent for the experiments. Once everything cooled, the metal crystals formed, but the gallium remained liquid. They were able to extract the metal crystals by reducing the surface tension of the gallium solvent – achieved via a combination of electrocapillary modulation and vacuum filtration – and carefully documented the different morphologies of each.
They then performed molecular dynamics simulations to determine why different metals produced crystals with different shapes: cubes, rods, hexagonal plates and, in the case of zinc, a snowflake structure. They found that it all comes down to the interactions between the atomic structure of the metals and the liquid gallium. “What we’re learning is that the structure of the liquid gallium is very important,” Gaston said. “That’s new because we usually think of liquids as having no structure or just being randomly structured.”
DOI: Science, 2022. 10.1126/science.abm2731 (About DOIs).
Frame image by Waipapa Taumata Rau/University of Auckland

